The story of the Sacklers – and the family’s involvement in pharmaceuticals – goes back to Brooklyn in the early 20th Century.
Three brothers – Arthur, Mortimer and Raymond – were born to Ukrainian and Polish immigrants. All three eventually made their way to university, studying medicine, and all ultimately became involved in psychiatry.
But during his studies Arthur also began working with pharmaceutical companies – through his role as a copy-writer for medical advertising agency William Douglas McAdams. That began as a way for him to earn money while in college, but he eventually went on to take control of the firm, and completely transform it.
And this is a crucial part of the Sackler origin story, because it’s here that he developed a skill for marketing medicines.
He helped to turn Pfizer from what was then a chemical company into a medicines company, and he played a major role in making Valium a hugely popular drug in the US. It was the first drug to generate $100m in sales back in the 1960s.
Part of his successful approach was the leveraging of the medical newspaper he had bought – The Medical Tribune. That was popular with doctors and became a key tool for drug companies to raise awareness around their products.
Arthur was also a pioneer in direct marketing to doctors – the idea of sending sales reps out around the country to encourage GPs to prescribe their product.
And after years of working in medicine, and helping drug companies to sell their products, Arthur decides to take on yet another role. That leads he – and his two brothers – to acquire Purdue Pharmaceuticals in the early 1950s. It had been going since the 1890s, and was a relatively small player, but Arthur & Co clearly had bigger plays.
That began to take shape in the late 1960s, when Purdue acquires another drug company – Knapp Laboratories. This marks the start of the company’s move towards treating pain management.
So where does that start?
One of its first and most popular medicines in this area was called MS Contin. It was a time-release morphine – so it promised to give relief for eight to 12 hours.
At the time it – like regular morphine – was mainly being used to treat pain management in cancer patients. And it proved to be a fairly popular product for Purdue – helping it to grow its revenues.
But the fact that it was a relatively niche product is a problem for the Sacklers – because it limits their market. And they start to think about what other applications a pain management drug could have.
Why would you only need pain management for cancer patients, they think, when there are plenty of people living with mild or severe pain from old injuries, or wear and tear from physical labour?
But while they are looking to expand, they are also facing a significant problem.
Because medical patents only last 20 years which means that, by the late 1980s, MS Contin was on the verge of coming off patent. That would allow any company to make a copycat drug – likely selling it at a lower cost – which would present an existential threat to Purdue’s bottom line.
And it’s at this point that they start to look for an alternative – which is when Oxycontin is developed.
It’s based on an opioid called oxycodone – which is stronger than morphine.
And, like MS Contin, one of the selling points of Oxycontin is that it’s slow release. This, they say, means the user doesn’t get a spike of relief that quickly wears off – and it’s effects will last for hours.
And this becomes an important point in the approval process of the drug.
Why?
When creating any new medicine, you need approval from the relevant authority before you can start to offer it to patients.
In Ireland there’s the European Medicines Agency and then the Health Products Regulatory Authority. In the US it’s the Food and Drug Administration, or the FDA.
In the case of Oxycontin and its approval process (which is handled by the FDA’s Curtis Wright) this slow release process is seen to be a protection against addition – and a disincentive for abuse. Because, the theory goes, patients are not going to get the same buzz from taking this as they would other types of drug that have an immediate, strong effect.
But some serious flaws in the FDA process have come to light in recent years.
For example, there were no clinical tests done to actually try to figure out whether the drug was more or less addictive, or open to abuse, than other opioids. That claim was just taken on face value.
That even led to a line in the approval documentation that said “delayed absorption… is believed to reduce the abuse liability of a drug”… This was something Purdue made a big deal of in its marketing material – as it helped to reassure doctors and patients. But Wright said it was essentially just an opinion, rather than something that was proven.
One thing a closer examination might have uncovered was the fact that the slow release mechanism was easy to get around – because it was the coating on the pill that made it slow release. All users had to do was crush it or bite it, and they would feel the drug’s effects much faster than intended.
In the book ‘Empires of Pain’, Patrick Radden Keefe discovered that a number of people working for Purdue actually spent time with Wright to help him work through his analysis of the studies they provided as part of their approval application.
So there seemed to have been, at best, a very thin line between the pharmaceutical company and the regulator.
And a lot of people point to the fact that Wright left the FDA after Oxycontin was approved, and was hired by Purdue a year later, as further proof of a cosy relationship.
So Oxycontin gets approved – how long before it becomes a so-called blockbuster drug?

Not very long, really.
Oxycontin gets approved right at the end of 1995, it hits the market in 1996 – and within five years, it’s brought in revenues of $2.8 billion for Purdue Pharma.
And a big part of that is down to that direct marketing approach that Arthur Sackler had perfected with other pharma companies.
Purdue employed hundreds of sales reps to go around the country, and encourage doctors to prescribe the drug. And they used that FDA approval – and the suggestion that it had a reduced risk of addiction and abuse – in their pitch.
They wanted this to be a drug that would be of use to anyone with any kind of pain issues – not just people who were seriously ill. And, as anyone who has ever watched US TV will know, pitching your prescription product to a wide audience is much easier there than it is in Europe.
Ad breaks are chock full of ads for prescription drugs, which make it seem like every little niggle and imperfection can be fixed with a pill. They tend to end with the line “ask your doctor if Oxycontin is right for you”.
That’s a way of getting patients to put pressure on their GPs to get some kind of prescription – and this drug in particular – rather than just asking them for the best treatment for a problem.
It’s also worth bearing the high cost of US healthcare in mind. That means you have so many people who are carrying niggles and injuries, some of which might require a relatively simple physio or surgery, but getting that done is prohibitively expensive.
That makes a cheap drug that promises to solve pain problems particularly attractive.
Purdue also pushed the fact that it offered 12 hours of relief, which they knew would make it more attractive than the alternatives. But in reality they knew it didn’t – it was probably closer to eight hours of relief. Or possibly even less.
To counter this they encouraged doctors to prescribe a stronger dose than was initially indicated.
And it worked.
The year Oxycontin hit the market, there were around 8 million opioid prescriptions in the US.
That had risen to 89 million by 2010, and 123 million by 2023.
Of course not all of those are Oxycontin – there were and are other opioids on the market, and other pharmaceutical came along with new drugs to compete with Purdue. All of which have ultimately contributed to the problem.
Why did the doctors go along with this?
This medicine, and others, did have the FDA approval behind it – so that gave it credibility.
And the sales people did a good job of playing up the positives, and playing down the negatives.
Because, on paper, this was a simple and quick fix to the problems patients were facing.
But it also transpired years later that those Oxycontin sales reps – who were incentivised to get more doctors to prescribe through bonus schemes – were in turn incentivising doctors in different ways.
Some of that was with freebies – or even invites to medical conferences that happened to be taking place in lovely resorts. In extreme examples the big prescribers – or ‘whales’ as they described them – were also paying doctors tens and even hundreds of thousands of dollars to give speeches at Purdue-organised events.
That focus on high prescribing doctors was in part motivated by a recommendation by consultancy firm McKinsey, which was brought on board by Purdue to help further boost sales.
How long before people realise it’s creating problems?
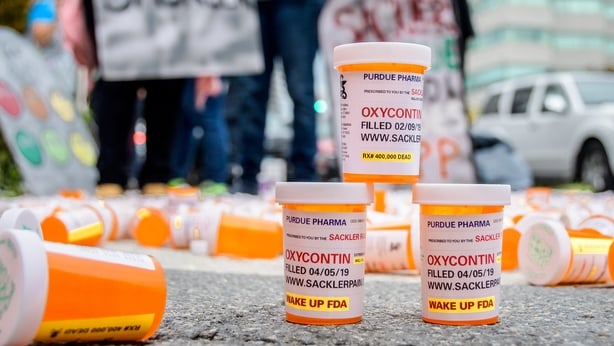
The Sacklers claimed that they didn’t know the drug was creating any problems until 2000. That was when the US Attorney for Maine wrote to doctors about issues around people overdosing – and a rise in crime related to the drugs.
But the truth is that they knew long before that. In fact the problems became apparent to people on the front lines quite quickly.
Those sales reps who were talking to doctors – they were hearing first-hand about people getting addicted, they were hearing about break-ins at pharmacies, and they were hearing about deaths. And they were reporting that back to their bosses.
In recent years emails have emerged showing senior executives at Purdue talking about the abuse problem, and talking about the ‘street value’ of Oxycontin – long before 2000.
Those emails also showed that they knew GPs were under the impression Oxycodone was weaker than morphine – even though it was stronger – but they told their reps not to correct them.
And they were encouraging doctors to prescribe heavier doses – because that meant they were selling more drugs, and making more money.
And the truth is there were lots of doctors who kept prescribing – and pharmacies dispensing – long after the problems started to emerge, in no small part because of the incentives Purdue built into their business model.
But it’s not until 2007 before there is finally a legal ruling against Purdue – and an admission by the company that its staff may have misled regulators, doctors and customers.
But the drug keeps selling…
Yes – the 2007 case was very much focused on the branding and marketing material – it was found to have ‘mis-sold’ the drug.
But there still wasn’t really a full reckoning with just how dangerous the drug – and others like it was.
And so it continued to generate billions for the company.
Purdue Pharma is privately-owned so it’s hard to get exact figures on how much it earned from Oxycontin – it doesn’t publish annual results like a listed company.
But from the various investigations and court cases, it’s thought the drug made a total of $35 billion for Purdue by 2017.
It’s thought that the Sacklers themselves made somewhere in the region of $11-13 billion from the company.
Does this settlement bring an end to the saga?
Not really – the Sacklers have made previous attempts at wrapping this up, for example they tried to push through a previous settlement offer that would have given them immunity from any further prosecution – while also putting Purdue into bankruptcy.
But that was rejected by US courts – and so this new settlement leaves the door open for people to take separate cases. So this has the potential to drag on for many more years.
That’s particularly true because the Sacklers have made clear they will fight any cases taken against them – and they have never fully accepted responsibility for the harmful effects of the drug, and the way it sold it.
At the same time there are thought to have been more than 700,000 opioid-related deaths in the US – and many more who suffered serious addiction.
There was also a 2012 study which suggested that three quarters of people seeking help for heroin addiction began by abusing prescription painkillers like Oxycontin.
So the harm caused by these drugs is likely to be felt for a very long time.
The Sacklers have also been accused of ‘reputation laundering’ – what does that mean?

For a long time, the Sacklers have been major philanthropists.
They’ve donated to the likes of the Guggenheim, the Met, Yale, Harvard and Cornell – as well as the Lourve, the Tate Modern, Kings College in London.
And they’ve had all sorts of buildings and wings of buildings named after the family over the years.
Many suggest this was part of an attempt to make the family appear charitable and kind – to distract from the harm its company was doing in the name of profit.
And despite the increased focus on that harm in recent years, those donations have continued. The British-registered Sackler Trust pledged more than $6m in 2022… though nowadays organisations are less willing to admit that they took the money than might have been the case before.
Another interesting bit of reputation laundering that the Sacklers have attempted has been to completely distance themselves from Purdue Pharma.
It’s been family-owned for generations now – which is usually something companies like to brag about. But in the case of the Sacklers and Purdue, they’ve kept their name away from the company’s marketing and publications. And despite all their charity work, they’ve also avoided mentioning where that donation money was coming from.